Image


In the nationwide recall of Artificial Tears Lubricant Eye Drops distributed by EzriCare and Delsam Pharma, an incorrect consumer contact phone number is being given to consumers, Westwood Minute has learned from a resident of Norwood, Massachusetts, who is receiving those misdirected calls at her place of work for a local company.
The Norwood resident, who asked to be identified as Deb M., said that the last digit of the Delsam Pharma recall contact phone number that is widely being reported by media, 1-866-826-1309, is incorrect. Dialing with the last digit “9” results in phone calls being directed to her place of work.
When Westwood Minute called this phone number on Saturday evening, February 4, 2023, a recorded message stated that a company called “Middlesex Overhead Doors” had been reached, not EzriCare or Delsam Pharma. Middlesex Overhead Doors is a garage door company with an office in Norwood, Massachusetts.
The correct phone number ends in the last digit “6,” says Deb M. The correct number would appear to be 1-866-826-1306. This is the phone number that appears at the bottom of the Delsam Pharma home page. Westwood Minute attempted to contact this number on Saturday evening before posting this article, but there was no response, possibly due to the call being made outside business hours. However, there was also no option to leave a message.
Deb M. says she began receiving recall inquiries at her place of work at the garage door company on Friday, February 3rd. Although she has lost track of the exact number of calls, she says she must have received at least twenty phone calls. She has been directing those callers to the correct phone number.
There may be more consumers calling the wrong Delsam Pharma number. Deb M notes that along with herself, a number of different employees are responsible for answering phone calls at the garage door company.
The incorrect phone number has been widely disseminated in media reports about the recall. It appears as the contact number on the Federal Drug Administration’s (FDA’s) website under information titled, “Company Announcement, Global Pharma Healthcare Issues Voluntary Nationwide Recall of Artificial Tears Lubricant Eye Drops Due to Possible Contamination.”
 Image capture from OTC Recall link download from Global Pharma Healthcare homepage on 2/5/2023. The incorrect Delsam Pharma number appears in recall information from Global Pharma Healthcare's website.
Image capture from OTC Recall link download from Global Pharma Healthcare homepage on 2/5/2023. The incorrect Delsam Pharma number appears in recall information from Global Pharma Healthcare's website.Global Pharma Healthcase also published the incorrect Delsam Pharma recall phone number on the company’s website in a February 1, 2023 “OTC Product Recall” press release.
The incorrect Delsam Pharma phone number also currently appears on the web page of the National Institute of Health. In a “DailyMed” product description of Delsam Pharma eye drops, the incorrect number is published as the phone number to call with “questions or comments.”
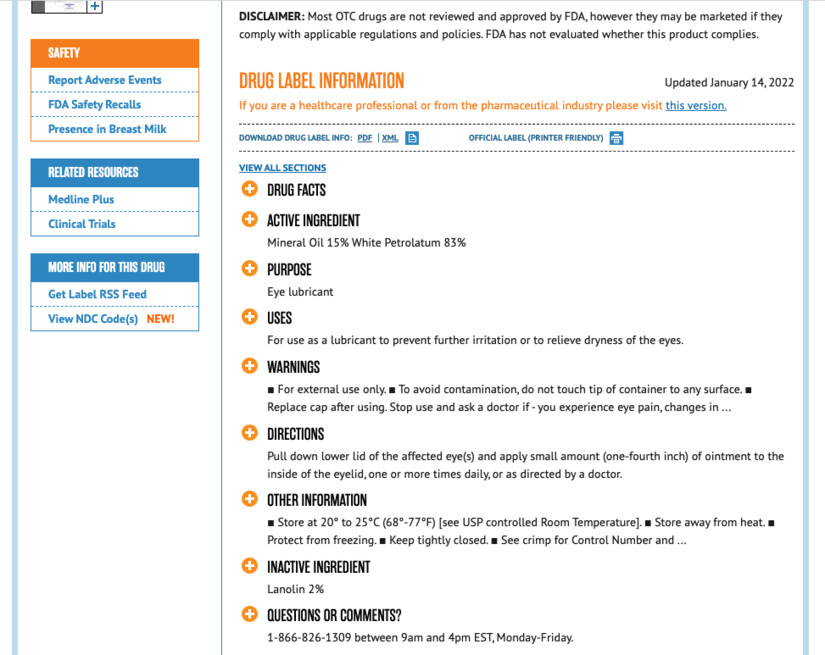 Image capture from dailymed.nim.nih.gov. The incorrect Delsam Pharma phone number appears at the bottom of this screen grab from February 5, 2023.
Image capture from dailymed.nim.nih.gov. The incorrect Delsam Pharma phone number appears at the bottom of this screen grab from February 5, 2023.The voluntary company recall for the artificial tear drops was issued following a Centers for Disease Control (CDC) health alert of February 1, 2023.
On February 1st, CDC advised health professionals that an “extensively drug resistant strain” of bacteria was being found in eye infections of patients who used artificial tears. The majority of those patients reported using EzriCare Artificial Tears, said CDC.
One day later, FDA warned consumers not to purchase or use EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears. Both products are preservative free and are both manufactured by Global Pharma Healthcare Private Limited.
FDA noted that Global Pharma had violations of good manufacturing practices, including lack of appropriate microbial testing and lack of control over package tampering.
Update 2/8/2023: As of February 8th, following Westwood Minute's alert and communications with the FDA and Delsam Pharma, the incorrect phone number no longer appears in the February 2nd recall notice. It has been corrected.
Thanks to Deb M. for speaking with Westwood Minute.